Microglia And Bipolar.
Recent investigations suggest that microglia, the resident immune cells of the brain, provide a physiological link between the serotonin system and the GSK-3β/Wnt pathway through neuroinflammation.
In clinical practice, patients that fail to respond to an optimized regimen of mood stabilizers tend to respond to augmentation with selective serotonin reuptake inhibitors (SSRIs). Bipolar depression mistaken for major depressive disorder (MDD) and treated with SSRIs can cause a ‘switch’ effect where patients may become manic. Although the serotonin system in particular has been implicated in the pathophysiology of bipolar depression, a growing body of evidence is shifting from theories of simple deficits in serotonin to those based on effects secondary to abnormalities in neuronal plasticity. In this context, plasticity is defined as an experience-dependent change in synaptic strength, cell resilience and/or connectivity.
Homeostasis, support and protection of neurons are maintained by glia cells (microglia and astrocytes) in the brain and peripheral nervous system. Although astrocytes are large stellate cells that provide biochemical support, microglia are specialized macrophages capable of phagocytosis in order to protect neurons in the central nervous system (CNS). Microglia appear to function as sensors and regulators of serotonin production through pro-inflammatory cytokines in the brain. One link between microglia and serotonin production may be inflammatory signaling in the kynurenine pathway (KYP), an alternate route of tryptophan metabolism that decreases serotonin neurotransmission.
Changes in the cytokine environment and serotonin production mediated by microglia appear to lead to long-term changes in synaptic function and downstream effects of apoptosis, excitotoxicity, neurogenesis and neurotrophic production. Developmental, neuropathological and imaging data in basic science and clinical models of BD demonstrate that serotonin production and neuronal connectivity are dependent on immune cytokine pathways in the periphery and the brain.
Mood stabilizers such as lithium (Li) and valproic acid (VPA) are the first-line pharmaceutical agents used in both the depressed and manic phases of BD. Although the mechanism of action of Li and VPA are unclear, they both appear to reduce immune cell signaling through inactivation of the enzyme glycogen synthase kinase-3 beta (GSK-3β) in the Wnt pathway. Li and VPA also inactivate other enzymes involved in inflammation, including cyclooxygenase 2 and aracadonic acid.
Serotonin and bipolar depression.
Serotonin is involved in mood, sleep, appetite and energy level, all of which are altered in BD. The illicit drug 3,4,-methylene-dioxy-methamphetamine (ecstasy) inhibits the reuptake of serotonin through the serotonin transporter (5-HTT). In a mood state that resembles BD, the transient elevation in serotonin activity generates feelings of elation, followed by depressive feelings related to depleted serotonin when the drug wears off.
Monoamines have been implicated in altered production, transport, storage, release, reuptake and degradation of neurotransmitters in BD. Excitatory N-methyl-D-aspartate receptor and inhibitory gamma-aminobutyric acid amino acids, cholinergic and noradrenergic systems, corticotrophin releasing factor, thyrotropin releasing factor and other neurotransmitters and neuromodulators have been linked to the depressive phase of BD. SSRIs, in the form of commonly prescribed antidepressant drugs, have hel ed to expand our knowledge of both unipolar depression (MDD) and BD and the mechanisms behind these disorders.
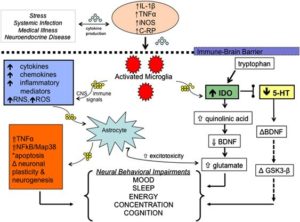
Multiple animal studies suggest that serotonin signaling may be influenced by immune cytokine pathways in the brain and periphery through the KYP. Activated microglia in the KYP trigger an alternate route of tryptophan metabolism that ultimately reduces overall serotonin availability in the brain. Cytokines such as IL-2 and IL-6, released in the periphery from macrophages and interferon-gamma (IFN-γ) from activated microglia within the CNS, may provoke overstimulation of the KYP that effectively depletes CNS serotonin levels and alters trophic support from brain-derived neurotrophic factor (BDNF) and TNF-α.
In BD a decreased plasma ratio of tryptophan, the precursor to serotonin, to other large, neutral amino acids was demonstrated in acute mania. Those serotonin deficits in the manic phase of BD form the basis of an interesting hypothesis that overstimulation of the enzyme indoleamine 2,3-dioxygenase (IDO) by peripheral and central inflammatory mediators activates the consumption of tryptophan in the KYP (Figure 1) and leads to serotonin depletion. That pathway represents an alternative metabolic route for tryptophan. Activated microglia in the brain promote expression of cytokines that leads to increased IDO activity and CNS tryptophan depletion with a concurrent increase in brain quinolinic acid, a metabolite produced distally within the KYP.
Based on pharmacological evidence, microglia appear to have a functional role in serotonin neurotransmitter signaling through inflammatory mechanisms that ultimately affect the Wnt/GSK-3β pathway and mood in BD. Glial cells have become increasingly important in understanding neuroplasticity and cellular resilience mechanisms.