Stress and Inflammation.
Stress is a state of threatened homeostasis provoked by a psychological, environmental, or physiological stressor. With rapid development of science and technology, as well as economy and strong social competition, the nature of stress has changed dramatically.
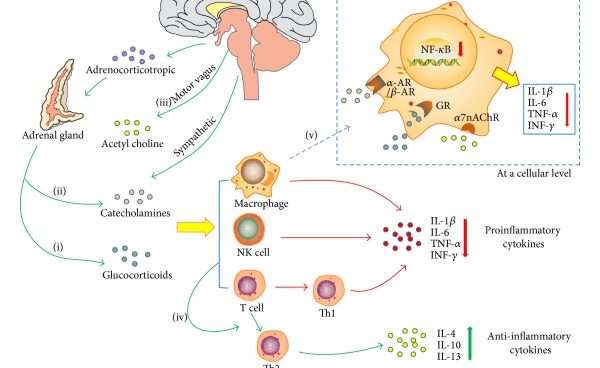
Stressful events engender multiple neurochemical, neurotransmitter and hormonal alterations by mainly activating the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. When stress stimuli are under control, the body responds to these in the physiological way. SNA and HPA axis are woken up to release chemical mediators to protect our body from stress. For instance, catecholamines are elevated to increase heart rate and blood pressure, which help us to fight or flight. This appropriate body reaction was called “allostasis” by Sterling and Eyer. This state is beneficial to our survival and recovery. However, when stress stimuli are prolonged or over exaggerated, in another word, chronically increased allostasis lead to pathophysiology.
In the last two decades, accumulating evidence indicated that severe or prolonged (chronic) stress resulted in increased risk for physical and psychiatric disorders, which is called stress-related disease. Stress is the common risk factor of 75%–90% diseases, including the diseases which cause the foremost morbidity and mortality. According to the former review, the most common stress-related diseases are cardiovascular diseases (CVD, i.e., hypertension and atherosclerosis), metabolic diseases (i.e., diabetes and non-alcoholic fatty liver disease, NAFLD), psychotic and neurodegenerative disorders (i.e., depression, Alzheimer’s disease, AD and Parkinson’s disease, PD), cancer.
Large bodies of evidence indicate that stress can activate inflammatory response in brain as well as peripherally.
There exists communication between the neuroendocrine and immune systems. Stress activates the HPA axis through the hypothalamic secretion of corticotropin-releasing hormone (CRH), which normally suppresses immune responses through the release of glucocorticoids (GCs) from the adrenals. GCs are one of the major stress hormones released during stress response that are well known for their immunosuppressive and anti-inflammatory properties.
Both pro-inflammatory and anti-inflammatory mechanisms depend on the type and intensity of stressors. Acute stressors seem to enhance immune function, whereas chronic stressors are suppressive. Intense stressors over-activate the immune system, leading to the imbalance of inflammation and anti-inflammation. Reports from different labs have confirmed pro-inflammation induced by stress, including C-reactive protein (CRP), IL-6, TNFα, IL-1β and the transcription factor of “nuclear factor kappa B (NF-κB)”.
In addition to peripheral inflammation, central inflammation namely neuroinflammation, has also been found in stress condition. Elevated pro-inflammatory cytokines, increased microglia activation and accumulation of peripherally-derived monocytes and macrophages were detected in the brain with psychological stress exposure. As the brain-resident macrophages, microglia was considered to be the major pro-inflammatory cytokine source. Stress-elicited potentiate microglial activation is via both direct and indirect mechanisms.
Microglia express both GC and mineralocorticoid receptors, thus microglia are likely to have direct response to corticosterone peak.
In addition, GC receptors also are highly present in the hippocampus and prefrontal cortex, so stress-induced corticosterone may have indirect effects on microglia.
Besides this, a recent research display that CNS innate immune system can respond to an acute stressor, thereby releasing the danger signal high mobility group box-1 (HMGB-1) in the brain to prime microglia by acting on the NLRP3 inflammasome, in preparation for IL-1β secretion. Activated microglia display hypertrophic branch morphology with an enlarged soma and produce an exaggerated cytokine to recruit peripheral monocytes. Increased brain macrophages and circulating monocytes, contribute to elevated levels of pro-inflammatory cytokine production (i.e., IL-1β, TNFα, IL-6) in the brain