Risperidone.
Current practice guidelines for the treatment of bipolar mania support the use of atypical antipsychotic medications as monotherapy or as a component of polytherapy, and in clinical settings the use of atypical antipsychotics to treat bipolar disorder is widespread.
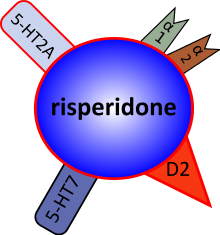
Risperidone is an atypical antipsychotic, sometimes referred to as a second-generation antipsychotic. The receptor-binding profile of risperidone, which includes potent antagonism of the serotonin 5-HT2A, dopamine D2, and alpha-adrenergic receptors, is believed to be related to positive effects on mood.
The FDA-approved bipolar indications for risperidone include: 1) monotherapy for short-term treatment of acute manic or mixed episodes associated with bipolar I disorder and 2) combination therapy with lithium or valproate for the short-term treatment of acute manic or mixed episodes associated with bipolar I disorder.
For bipolar mania in particular, the atypical antipsychotics have demonstrated efficacy and generally good tolerability. A recent report based upon a large US registry of over 65 000 individuals with bipolar disorder noted that in 2001, more than 40% of individuals with bipolar disorder were prescribed antipsychotic medications, and that 85% of these were on atypical antipsychotics.
Risperidone is a selective monoaminergic antagonist with high affinity (Ki 0.12–7.3 nM) for the 5-HT2, D2, alpha1, and alpha2 adrenergic and H1 histaminergic receptors. It has low to moderate affinity (Ki 253 nM) for the serotonin (5-HT1c, 5-HT1d, and 5-HT1a) receptors, weak affinity (Ki 620–800 nM) for the dopamine D1 and the haloperidol-sensitive sigma site, and no affinity for cholinergic muscarinic or B1 and B2 adrenergic receptors.
Risperidone is well absorbed. Food does not affect the rate and extent of absorption. The absolute oral bio-availability of risperidone solution is 70%. Bioavailability of the oral tablets as well as the orally disintegrating tablets is 66%. Plasma concentrations of risperidone, 9-hydroxyrisperidone (its major active metabolite), and the combination of risperidone and 9-hydroxyrisperidone are dose proportional over the dosage range of 1–16 mg/day. Peak plasma concentrations occur at about 1 hour in extensive metabolizers and 3 hours in poor metabolizers.
The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity to risperidone. Therefore, the clinical effects of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone (active moiety).
The half-lives of risperidone and 9-hydroxyrisperidone are 3 hours and 21 hours, respectively, in extensive metabolizers, and 20 hours and 30 hours, respectively, in patients who are poor metabolizers. The overall mean elimination half-life of the active moiety is approximately 20 hours.
Risperidone is available as a standard oral tablet, a fast-dissolving tablet, and an oral solution, as well as a long-acting injection. Alternatives to a standard oral formulation may be of particular use in situations where patients may have difficulty swallowing pills or where medication adherence is a concern. The long-acting dosage formulation uses the extended-release drug-delivery system: small polymeric microspheres degrade slowly, releasing the medication at a controlled rate. The long-acting injection of risperidone exhibits different pharmacokinetics from the standard oral formulation. After a single intramuscular injection there is a small initial release of drug (< 1% of the dose), followed by a lag time of 3 weeks and subsequent release of risperidone. Therefore, oral antipsychotic supplementation should be given for at least 3 weeks after the administration of the initial intramuscular injection of long-acting risperidone to maintain therapeutic levels until the main release of risperidone from the injection site has begun.
Risperidone, a CYP2D6 substrate, could be subject to 2 kinds of drug–drug interactions. First, inhibitors of CYP2D6 interfere with the conversion of risperidone to 9-hydroxyrisperidone. Examples of drugs that inhibit CYP2D6, and therefore risperidone metabolism, include fluoxetine and paroxetine. Combined use of risperidone and one of these SSRIs will require the clinician to review risperidone dosing and the patient’s clinical status. A dosage adjustment may be needed. The second type of drug–drug interaction could result from co-administration of risperidone with a known enzyme inducer such as carbamazepine, phenytoin, rifampin, or phenobarbital. This co-administration could result in a decrease in the combined plasma concentrations of risperidone and 9-hydroxyrisperidone. For example, plasma concentrations of risperidone and 9-hydroxyrisperidone may be decreased by 50% with concomitant use of carbamazepine. If carbamazepine is initiated a dosage increase may be needed. Conversely, if carbamazepine is discontinued, a decrease in dosage may be needed. Of relevance to the treatment of patients with bipolar disorder, risperidone does not appear to interact with lithium or valproate.
Recommended dosing of risperidone in bipolar mania is 2–3 mg once daily. In more severely ill hospitalized patients such as those included in pivotal trials higher doses may sometimes be required. If needed, this may be adjusted by 1 mg/day in intervals of approximately 1 day. Suggested dosing range is 1–6 mg/day in healthy individuals. In the elderly, a starting dose of 0.25–1 mg in 1–2 divided doses is recommended, with slow titration if needed. If once-daily dosing in elderly or debilitated individuals is considered, a twice-daily regimen should be used to titrate to target dose, and this dose maintained for 2–3 days prior to switching to once-daily dosing.
Common side effects include movement problems, sleepiness, trouble seeing, constipation, and increased weight. Serious side effects may include the movement disorder tardive dyskinesia, as well as neuroleptic malignant syndrome, an increased risk of suicide, and high blood sugar levels. In older people with psychosis as a result of dementia, it may increase the risk of dying.
With Risperdal, a common off-label use is in elderly patients who suffer from dementia. The FDA has placed a black box warning on the medication indicating that using Risperdal for this purpose is associated with an increased risk of death.
Risperidone acts on the following receptors:
Dopamine receptors: This drug is an antagonist of the D1 (D1, and D5) as well as the D2 family (D2, D3 and D4) receptors, with 70-fold selectivity for the D2 family. This drug has “tight binding” properties, which means it has a long half-life and like other antipsychotics, risperidone blocks the mesolimbic pathway, the prefrontal cortex limbic pathway, and the tuberoinfundibular pathway in the central nervous system. Risperidone may induce extrapyramidal side effects, akathisia and tremors, associated with diminished dopaminergic activity in the striatum. It can also cause sexual side effects, galactorrhoea, infertility, gynecomastia and, with chronic use reduced bone mineral density leading to breaks, all of which are associated with increased prolactin secretion.
Serotonin receptors: Its action at these receptors may be responsible for its lower extrapyramidal side effect liability (via the 5-HT2A/2C receptors) and improved negative symptom control compared to typical antipsychotics such as haloperidol for instance. Its antagonistic actions at the 5-HT2Creceptor may account, in part, for its weight gain liability.
Alpha α1 adrenergic receptors: This action accounts for its orthostatic hypotensive effects and perhaps some of the sedating effects of risperidone.
Alpha α2 adrenergic receptors: Perhaps greater positive, negative, affective and cognitive symptom control.
Histamine H1 receptors: effects on these receptors account for its sedation and reduction in vigilance. This may also lead to drowsiness and weight gain.
Though this medication possesses similar effects to other typical and atypical antipsychotics, it does not possess an affinity for the muscarinic acetylcholine receptors. It was recently found that D-amino acid oxidase, the enzyme that catalyses the breakdown of D-amino acids (e.g. D-alanine and D-serine — the neurotransmitters) is inhibited by risperidone.